|
|
|
Question 1
The easiest of these types of problems are those where you are given the concentration of each species at equilibrium and then you have to substitute those into the expression for the equilibrium constant. There are samples of these in the text so I won't do them here.
3 moles of ethanol were mixed with 3 moles of ethanoic acid in a 1 L vessel and the reaction was allowed to reach equilibrium at 80°C
At equilibrium, it was found that 2 moles of each product had been formed.
Write down the expression for the equilibrium constant and calculate it’s value at 80°C.
Notice the temperature has been included. This is because equilibria (and their constants) change at different temperatures, so a temperature must always be specified. However, you do NOT need to use the temperature in your calculation.
This is a simple one because the coefficients in the balanced equation are all 1 - so no higher powers in our K expression.
Now, don’t be in a rush to use the 2 mol for each of the products and 3 mol for each of the reactants. 2 mol for the products is OK but those 3 mol quantities were the starting amounts and, by now, some of this has been used up. So…the next step is to calculate the amounts present AT equilibrium.
To do this, use the amounts of product formed to calculate how much reactant was used up…I’ll use water as the product because it’s easier to type. It works with either one!
Finally, we can substitute these values into the expression for K to get
Question 2
Carbonyl bromide, COBr2, dissociates at 75°C according to the equation
Carbonyl bromide (2.00mol)was placed in a 2.00L container at 75°C and, after a period of time at this temperature, the amount of COBr2 in the container was found to be constant at 1.28 mol. Determine the value of the equilibrium constant for the reaction at 75°C.
Question 3
Hydrogen, used for the synthesis of ammonia, can be made according to
Equal number of mole of methane and water were added to an empty 1000cm³ reaction vessel at 1030K in the presence of a nickel catalyst. After the system reached equilibrium, the concentrations of methane and water were each 0.012M and the concentration of carbon monoxide was 0.0083M.
If the total pressure of the system was increased by decreasing the volume of the vessel, how would this affect the yield of hydrogen? Why?
If the total pressure of the system was increased by adding argon(an inert gas) to the vessel without changing its volume, how would this affect the yield of hydrogen? Why?
Question 4
An equilibrium mixture of NO2 and N2O4 is cooled in the container at a fixed volume. The colours of each gas are shown below
At a particular temperature, the equilibrium concentration of nitrogen dioxide is 0.60M and that of dinitrogen tetroxide is 0.486M. The equilibrium constant at this temperature is
At a particular temperature, the pressure is increased by pushing in the piston. In order to maintain equilibrium, the ratio [NO2]/[N2O4]
What about the problems where you are given the quantities of the starting material?
![]()
Worked Answer
The general equation for equilibrium constants is
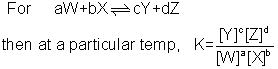
Remember, too, products go on top!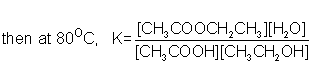
Mole ratio of H2O to ethanol is 1:1. therefore 2 mol H2O produced means 2 mol of ethanol used up. There were 3 mol of ethanol to start with so, at equilibrium, there is 3-2=1 mol left. Use the same reasoning to get 1 mol of ethanoic acid as well.
The equilibrium constant requires concentrations not moles, so using the container size, convert no. of mol to concentration. This is particularly easy when the vessel size is 1 L!
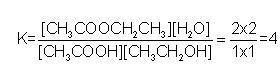
![]()
![]()
(a) What was the hydrogen concentration?
(b) Write the expression for the equilibrium constant and calculate its value at 1030K.
If more CO gas was added at 1030K, what would happen to the temperature of the vessel? Why?
![]()
As the mixture cools, the brown colour becomes fainter. The equilibrium constant has